Coffee is the most popular natural drink, which has a rich composition. It consists of numerous structural elements of organic and inorganic nature, affecting the human body in different ways. Freshly roasted coffee beans contain about a thousand different chemical compounds. So what does coffee contain and what effect does it have?
General information about the chemical composition
Coffee beans contain many biologically active components, but their concentration varies significantly depending on the method of preparing the raw material. These compounds are acidic or alkaline in nature depending on their structure.
Raw grains contain up to 12% liquid, about 8% sugary substances and the same amount of tannins. The share of fiber in fresh raw materials accounts for about a quarter of the dry residue. The alkaloid content in grains reaches 12%. Coffee beans necessarily contain fats, as well as proteins and carbohydrates in small quantities.
After roasting, coffee raw materials lose a significant amount of water, carbohydrates, and tannins. At the same time, the concentration of fats and alkaloids increases. Heat treatment promotes the storage of acids, vitamins and essential microelements.
How and from what instant coffee is made: production
Coffee comes in beans (or natural) and instant. About 50% of coffee beans are processed into instant coffee. It is prepared from 100% robusta, arabica and a combination of robusta and arabica. First, the coffee beans are roasted, then “allowed to rest” for 3-4 hours.
Then the grains are ground to a certain diameter - at least 0.5-1.1 mm and a strong stream of hot water is applied to them to wash out the carbohydrates from them. Cool, filter and thicken.
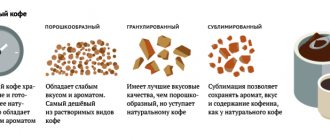
The resulting thick extract is dried using different methods:
- sprayed under hot air, obtaining the cheapest powdered coffee;
- by processing coffee powder with steam, granulated coffee is obtained;
- Freeze-drying produces freeze-dried coffee.
The last stage of coffee processing is aromatization: natural essential oils are added to expensive varieties, and flavorings are added to cheap varieties.
Content of proteins, fats and carbohydrates
Coffee beans, in addition to a huge number of active substances and aromatic compounds, contain three main groups of components:
- squirrels
- fats
- carbohydrates
Their ratio depends on the type of coffee, the method of preparation and the growing conditions of the plant.
Squirrels
The content of protein compounds strongly depends on the species of a particular coffee crop. The dependence of the concentration of alkaloids on the number of proteins was revealed. During storage, their quantity does not change in any way, but a tendency towards an increase in the water-soluble fraction is noticed.
In addition to proteins, the group of nitrogenous compounds includes more than 20 free-type amino acids. Their quantity does not change in any way during storage. High-grade coffee contains a predominant amount of them in its composition.
Fats
In coffee beans, the concentration of oily compounds depends on the species. The minimum amount of fat is found in coffee grown in India. Coffee oil has the peculiarity of a large number of esters. Saturated fatty acids in the oil make up more than half. The content of linoleic acid predominates.
The qualitative composition of the oily component is identical for all coffee crops. With increasing storage time, a tendency to slow down oxidative processes has been observed.
Carbohydrates
Complex and simple sugars in coffee beans have a strong influence on the taste of the finished drink. They can serve as precursors for the formation of volatile structures obtained during roasting. These compounds give the drink a special aroma.
Carbohydrates in coffee are found in the form of glucose, fructose, galactose and sucrose, the concentration of which is about 30% of the water-soluble fraction. Each type of coffee contains a certain amount of sugars. During storage, their concentration does not change much.
The composition of coffee beans includes fiber, which occupies almost a third of the volume; it gives density to the grain. This property does not allow the raw material to be severely destroyed or boiled, increasing in volume. Fiber helps preserve the aromatic compounds that give the drink its special smell. This compound is quite stable; different types of coffee plants do not differ in its content.
Products from Finland
Water
The moisture content of raw coffee is essential for assessing quality. The moisture content of raw coffee plays an important role in assessing its quality. This indicator is also important for export and import, since all payments between suppliers and buyers of coffee are made on the basis of this indicator. Raw coffee beans belong to the group of products with a capillary-porous colloidal structure. They are characterized by various forms of connection between water and the material (free, bound, tightly bound). The water content of raw coffee beans according to the standard adopted by the International Coffee Organization (IOC) should be 12±1%. The rate of sorption and desorption of water vapor by coffee beans is relatively high. Coffee beans absorb moisture especially intensively at elevated relative air humidity and storage temperature. At a relative air humidity of 40-60%, the moisture content in the grains does not exceed 12%. At air humidity of 63-65%, raw coffee retains normal color, freshness and taste throughout the year; at an air humidity of 65-70%, along with a yellow color, the characteristic smell and taste of stale coffee appears. When relative air humidity exceeds 75%, coffee acquires a musty smell and taste becomes almost unfit for consumption. At a relative air humidity of 95% and a temperature of 20-26 ° C, raw coffee beans reach equilibrium moisture content after 25-30 days, while roasted coffee after 5-7 days, and instant coffee after a few hours. Raw coffee is a biological object; the water it contains plays an active role in the biochemical and physicochemical processes that occur in the cells and tissues of the beans.
Extractives
The content of water-soluble extractive substances in different types and varieties of raw coffee varies and is approximately 20-29%. The smallest amount (19-20%) is found in the highest grade Arabica coffee. In the 1st grade of Arabica - 21-23%, and in the Kaniforma (Robusta) variety - 24-27%, in the 2nd grade of Kaniforma coffee - 27-29%. The extractive substances of raw coffee include alkaloids, proteins, phenolic compounds, mono- and disaccharides, lipids, organic acids, amino acids, mineral elements and a number of other substances contained in small quantities.
Caffeine
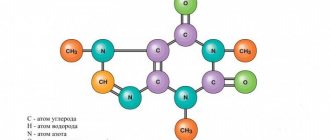
Caffeine (C8H10N4O2) is the most important alkaloid of coffee beans, known as 2,6-dioxy-1,3,7-trimethylpurine, or 1,3,7-trimethylxanthine:
This substance is colorless and odorless; in one solution it gives a bitter taste. Caffeine crystallizes from aqueous solutions in the form of crystalline hydrate, which has the shape of fragile silky needles. Anhydrous caffeine melts at 236.5ºC and can sublimate when heated carefully. It is easily soluble in chloroform, methylene chloride, di- and trichlorethylene. Aqueous solutions of caffeine have a neutral reaction; it forms salts with acids. Caffeine in raw coffee is in free and bound states with potassium chlorogen. Different types of coffee are characterized by the following caffeine content (% in terms of dry matter): Arabian - 0.6-1.2 Robusta - 1.8-3 Liberian - 1.2-1.5. The amount of caffeine in beans varies greatly depending on the type of coffee. The caffeine content in beans plays a very important role in assessing the quality of raw materials and establishing technical requirements for them.
Trigonelline
Trigonelline (C7H7O2N), or methyl betaine nicotinic acid, is formed in plants by methylation of nicotinic acid. This alkaloid is found in relatively large quantities in Arabica coffee varieties (1-1.2%). In varieties of the Caniforma (Robusta) type it is slightly less (0.6-0.74%), and in varieties of the Liberica type - only 0.2-0.3%. Trigonelline is highly soluble in water, but is thermally unstable. When processing coffee beans, it is easily converted into nicotinic acid (vitamin PP), so it is considered the main precursor for the formation of nicotinic acid in coffee beans.
Theobromine
Theobromine is a dimethylxanthine (C7H8O2N4), since upon oxidation it forms monomethylalloxane and monomethylurea. It is a colorless, finely crystalline powder, sparingly soluble in water. Theobromine melts at 351ºC, is capable of sublimation, and easily dissolves in caustic alkalis, giving, for example, sodium salt. The content of theobromine in raw coffee beans is insignificant - 1.5-2.5 mg%.
Theophylline
Theophylline is a 1,3-dimethylxanthine (C7H8O2N4) that forms colorless, silky needles containing one molecule of water of crystallization. Theophylline is sparingly soluble in cold water and melts at 269-272ºC. The total amount of it in beans of wild coffee plants is 1-4 mg%. From the groups of plant substances of secondary origin, the glucoside mascaroside (C12h26O11) was discovered and isolated in crystalline form in the beans of wild coffee plants (C. Vianneyi). It has been established that it is a pentacyclic diterpene glucoside, similar in some properties to cafamarin, isolated from coffee beans C. Buxifolia. No cafamarin was found in C. vianneyi coffee beans. Polyamines (putrescine, spermine, spermidine), which form various heterocyclic alkaloids upon deamination or oxidation, were isolated from raw grains and identified by thin-layer chromatography.
Chlorogenic acids
Chlorogenic acids constitute the main part of phenolic compounds. Chlorogenic acids are mono- and diesters of cinnamic and quinic acids. Esters of quinic acid with caffeic and ferulic acids were also found in coffee beans. Chlorogenic acid. It was first isolated in crystalline form from coffee beans by Gorter. Its structure was determined to be caffeoyl-3-quinic acid. Chlorogenic acids include about 10 compounds found in coffee, and similar compounds are found in other compounds. Isochlorogenic acid. It is actually a mixture of dicaffeylquinic acid. It consists mainly of three fractions of dicaffeylquinic acid and exists in the form of its isomers. Raw coffee beans contain approximately 7-10% chlorogenic acids. In coffee of the type Canifora (Robusta), their concentration is higher (9-11%) than in coffee of the Arabica type (5.5-8%). The main share of chlorogenic acids consists of caffeoylquinic acids (chlorogenic and non-chlorogenic). Thus, in Arabica coffee their content is 5.5-7%, and in Canifora coffee it is 8-9%. This is followed by dicaffeylquinic acids (isochlorogenic acids): in Arabica coffee there are 0.5-0.6% of them, in Canifora coffee - 1.4-1.7%. Coffee contains feruloylquinic acid in smaller amounts: Arabica coffee - 0.2-0.25%, Canifora coffee - 0.6-1.2%. The content of chlorogenic acids is determined by gas and thin layer chromatography. Using a colorimetric method, it was determined that the amount of tannins in Arabica coffee (India) is 6.1-6.36%, in first-grade Canifora (Robusta) coffee (India) - 6.8-7.7%, in first-grade Santos coffee. (Brazil) - 3.6-4.6%. During roasting, the content of chlorogenic acid in coffee beans decreases sharply - by 65-67%, cryptolorogenic acid - by 2 times, isochlorogenic acid - by 2.5-3 times. The decrease in the content of chlorogenic acids occurs due to their thermal destruction (the proportion of caffeic and quinic acids increases noticeably) and participation in reactions with amino acids and proteins with the formation of dark-colored products. The role of chlorogenic acids in the formation of coffee color during roasting is obvious.
Tannin
Tannin content in raw coffee beans varies widely - from 3.6 to 7.7%. During the frying process (especially at a temperature of 175-205°C), the amount of tannin decreases sharply and 0.5-1.0% remains in the finished product. This is a very labile component of coffee, which intensively oxidizes during 5-8 minutes of heat treatment at a temperature of 80-125°C. At this stage, polyphenol oxidase is active, which promotes the oxidation of tannin. Subsequently, a non-enzymatic transformation of tannin occurs, as a result of which secondary transformation products are formed - dark-colored pigments. The decrease in tannin content during roasting is not considered a negative factor, as it contributes to the formation of the taste and color of the coffee. However, with excessive heating, the tannin is completely decomposed. The hollow or flat taste of roasted coffee can sometimes be partially attributed to the disappearance of tannin. Therefore, taking into account the decomposition of chlorogenic acid, it is important to retain at least part of the phenolic compounds in the finished product. Using HPLC, LC/MS, GC/MS and UV spectroscopy, the content of phenolic acids in beans of 56 populations of wild coffee (Mascarocoffea) in Madagascar and 9 populations (Eucoffea) in Africa was studied. Ferulic and n-coumaric acids were found in most of the samples examined, and caffeic acid was contained in all samples. The main phenolic acids in Mascarocoffea coffee are o-coumaric, 3,4-dimethoxycinnamic and 3,4,5-trimethoxycinnamic. The content of sinapic and 4-methoxycinnamic acids is insignificant. Using Porter's reagents, the effect of 14-day air-drying of fruit pulp of three varieties of coffee from Venezuela (C. Arabica var. Red Bourbon, Red Catuai, Yellow Catuai) on the content of condensed tannins was studied. It has been proven that this indicator in fresh coffee pulp is 0.6-0.91%, and after drying - 0.88-1.19% in terms of dry matter.
Carbohydrates
Carbohydrates account for 50-60% of the total mass of raw coffee beans. Coffee carbohydrates include sucrose (6-10%), cellulose (5-12%), pectin (2-3%) and high molecular weight polysaccharides (fiber, lignin, etc.). It has been established that the main water-soluble component of high-molecular polysaccharides of raw coffee is arabinogalactan (2-5%). In addition, glucogalactomannan, galactose, mannose and arabinose have been isolated from coffee beans. For a long time it was believed that raw coffee lacks free monosaccharides (glucose and fructose), but research has found that sucrose predominates in Arabica coffee beans, and reducing sugars predominate in Caniforma (Robusta) coffee beans. Liquid chromatography in 80% aqueous solutions of ethyl alcohol of raw Arabica coffee beans from Ethiopia and Brazil, along with sucrose, revealed and quantified fructose, α-glucose, β-glucose and two unidentified sugars. In general, the total amount of reducing sugars in coffee beans reaches 0.7-1%. During the roasting process, profound changes occur in the composition of the carbohydrate complex of coffee. For example, sucrose, which is the main component of this complex, almost completely disappears (0.56% remains). At the beginning of frying, the monosaccharide content also drops sharply, but by the end of the process it increases significantly: 1.25% glucose, 1.1% fructose, 0.15% arabinose and 0.1% galactose. Fluctuations in the composition and amount of monosaccharides in coffee during its heat treatment are explained by the consumption of some of them for the processes of caramelization and melanoid formation (in the initial and middle stages of roasting), and then, when the temperature reaches 205-220°C, an increase in their concentration due to the hydrolysis of fiber , pentosans and other polysaccharides.
Protein substances
Raw coffee of the three main varieties (Arabica, Robusta and Liberica) contains proteins in almost the same amount (amine nitrogen - 1.55-1.63%, total protein content - 9.69-10.19%). The amino acid composition of raw coffee is studied using liquid ion exchange chromatography, and their quantity is determined by comparing the peak areas in the chromatogram of the studied samples, as well as the peak areas of the calibration mixture of amino acids. The separation and identification of coffee amino acids is also carried out using electrophoresis and thin layer chromatography. Coffee proteins contain 20 amino acids, including glutamic, aspartic, glycine and leucine. γ-aminobutyric acid was also found in coffee beans, and pipecolic acid was found in raw Arabica coffee beans and Arabica-Robusta hybrid coffee beans, which was not detected in raw coffee of other varieties. Liberica coffee beans do not differ in amino acid composition from other varieties of coffee. In general, it has been established that the amino acid composition of Arabica, Canifora and Liberica coffee is almost the same, but the content differs markedly, which is explained by the growing conditions. In roasted coffee, proteins contain the same composition of amino acids, but the amount of many of them is significantly reduced (serine - 3 times, glycine - 2 times, etc.). The total protein content is reduced by approximately 15%. Most likely, after roasting, coffee does not contain proteins, but protein-like substances, which are products of the interaction of proteins or their fragments with carbohydrates, phenolic compounds, etc. High levels of free amino acids have been found in raw coffee beans. Found over 1% phenylalanine, over 0.6% glutamic acid. But during the frying process, free amino acids disappear almost completely; they are detected only if there are traces of aspartic and glutamic acids, threonine, serine, and valine. It is obvious that free amino acids primarily enter into saccharoamine and quinone imine reactions, participating in the formation of color and the formation of coffee aroma. The German scientist Klechkus, having analyzed water-soluble melanoidins in coffee using liquid chromatography, found that their molecular weight ranges from 3,500 to 100,000. Moreover, the proportion of high-molecular-weight products of melanoid formation increased with increasing degree of roasting.
Lipids
Raw coffee of the three main varieties (Arabica, Robusta and Liberica) contains proteins in almost the same amount (amine nitrogen - 1.55-1.63%, total protein content - 9.69-10.19%). The amino acid composition of raw coffee is studied using liquid ion exchange chromatography, and their quantity is determined by comparing the peak areas in the chromatogram of the studied samples, as well as the peak areas of the calibration mixture of amino acids. The separation and identification of coffee amino acids is also carried out using electrophoresis and thin layer chromatography. Coffee proteins contain 20 amino acids, including glutamic, aspartic, glycine and leucine. γ-aminobutyric acid was also found in coffee beans, and pipecolic acid was found in raw Arabica coffee beans and Arabica-Robusta hybrid coffee beans, which was not detected in raw coffee of other varieties. Liberica coffee beans do not differ in amino acid composition from other varieties of coffee. In general, it has been established that the amino acid composition of Arabica, Canifora and Liberica coffee is almost the same, but the content differs markedly, which is explained by the growing conditions. In roasted coffee, proteins contain the same composition of amino acids, but the amount of many of them is significantly reduced (serine - 3 times, glycine - 2 times, etc.). The total protein content is reduced by approximately 15%. Most likely, after roasting, coffee does not contain proteins, but protein-like substances, which are products of the interaction of proteins or their fragments with carbohydrates, phenolic compounds, etc. High levels of free amino acids have been found in raw coffee beans. Found over 1% phenylalanine, over 0.6% glutamic acid. But during the frying process, free amino acids disappear almost completely; they are detected only if there are traces of aspartic and glutamic acids, threonine, serine, and valine. It is obvious that free amino acids primarily enter into saccharoamine and quinone imine reactions, participating in the formation of color and the formation of coffee aroma.
Organic acids and minerals
Among the organic acids found in raw coffee beans are citric, malic, maleic, acetic and oxalic. Moreover, for different types and varieties their composition and content are different. The acidity of raw coffee of various botanical species and varieties varies from 2.4 to 4°T. During long-term (3-5 years) storage of raw coffee under normal conditions, the acidity increases slightly. The content of free fatty acids in raw coffee beans of the highest grades is 0.5-3%, in beans of lower quality - up to 20%. The predominant ones are linoleic, palmitic and oleic acids. Raw coffee beans contain minerals. The content of individual mineral elements depends on the type of coffee, the growing area, the processing method, the type of mineral fertilizers applied to the soil, as well as the plant protection products used. There is no definite relationship between the amount of minerals and the quality of the coffee drink. However, it is believed that the zinc, manganese and rubidium content of raw beans is responsible for the best properties of the finished coffee. Raw coffee beans contain 3-4.5% mineral content. The predominant element is potassium (about half), followed by magnesium and calcium (about 10 times less), sodium, iron, manganese, etc. It is believed that the increased content of zinc, manganese and rubidium helps improve the properties of the drink. For example, the use of induction plasma atomic emission spectroscopy to study the kinetics of aqueous extraction of potassium, magnesium, manganese, and phosphorus from coffee samples is described. During coffee roasting, the mineral content increases to 5-7%, which is associated with large losses of dry matter. That's all. This seemingly simple drink - coffee, which we drink every day, turned out to be not so simple. In the next article I will talk about the technological process of preparing everyone’s favorite instant coffee.
Alkaloids
Coffee contains abundant amounts of alkaloids, but only some of them are capable of producing physiological effects. As the coffee bean matures, it accumulates two main alkaloids:
- caffeine
- theobromine
The first is stored in the shell of the product, the second in the inner part. If the drink is made from unprocessed whole grains, then both nitrogenous substances are present. Coffee alkaloids determine the tonic effect of the drink on the body.
Caffeine
This alkaloid is an important component of coffee. It is this that causes all the physiological effects caused by the tonic drink. In its pure form, this substance has no distinct odor or color, but it can be identified by its rather bitter taste. This substance has high solubility in polar liquids and a neutral reaction in the environment. In coffee, the alkaloid is found in monovariant or associated with sour potassium in salt-type compounds.
The concentration of the main nitrogenous compound determines the quality of coffee. Each type of this drink differs in its concentration of alkaloid. This is influenced by the natural conditions in which the raw materials were grown.
Caffeine is responsible for the main effect of the drink. This is primarily a stimulation of the nervous system. But with frequent use it can cause slowness in the conduction of nerve impulses. And in abundance it can provoke exhaustion of the body.
Theobromine
This nitrogenous compound, like caffeine, has a pronounced bitter taste. It differs from caffeine in its saturation with methyl groups. Theobromine is poorly soluble in aqueous media and occurs in the form of crystalline hydrate. This alkaloid is most often clear or white in color.
This substance acts almost like caffeine, but the effect is not as pronounced. Theobromine has a high affinity for the cardiovascular system. It has a positive effect on the functioning of the heart muscle, increasing its contractility and improving the functioning of the coronary arteries. The most pronounced effect is noticeable on the muscles involved in breathing. Theobromine also helps reduce blood pressure and reduce renal blood flow. For this reason, after taking a tonic drink, it is necessary to replenish your water balance.
Interesting facts about caffeine
Caffeine is odorless and colorless, and when dissolved in water it gives the drink a bitter taste. It is bitterness that can explain the taste of brewed coffee. Caffeine melts at a temperature of 236 degrees Celsius, and if it is subjected to gradual heating, it can even sublimate, that is, pass from a solid to a gaseous substance immediately, without turning into a liquid. In different types of coffee, caffeine can have varying degrees of content - from 0.6 to 3%. In addition to coffee beans, caffeine is found in large quantities in tea and coca leaves, as well as in mate and guarana. As for the medicinal properties of caffeine, it is known that it stimulates brain receptors, increasing motor activity and improving reactions. This is why many cyclists (and caffeine has recently been re-allowed in cycling) consume caffeine bars, which significantly improve their performance, during the race.
Taurine
Taurine is a substance with a structure similar to an amino acid. Antioxidants in coffee are represented by this substance. This compound is usually synthesized in the human body. Taurine in coffee can have a beneficial effect on the body.
Positive effects of this substance:
- pronounced antioxidant effect, which prevents carcinogenesis;
- decreased blood sugar levels;
- regenerative effect on the optical structures of the eye;
- hypotensive effect;
- participation in the metabolism of calcium, sodium and potassium;
- positive effect on the nervous system.
Chlorogenic acids
Phenolic compounds in coffee beans are represented by chlorogenic acids. Esters of cinnamic acids reach the highest concentrations. Esters of caffeic and ferulic acids are also found. These compounds are responsible for the acidity of coffee.
The content of these compounds in grain reaches 10%. Their content decreases during roasting as they are destroyed by temperature. Chlorogenic acids give beans their dark color after roasting. The greater their concentration, the darker the color.
Vitamins and microelements
Coffee made from beans of natural origin contains a large amount of vitamins and microelements. Vitamins in coffee are widely represented. They play an important role in human physiology. What vitamins are present in the drink:
- Niacin is a substance that regulates oxidation processes in cells. Helps normalize blood cholesterol levels and participates in the processes of synthesis of energy reserves. has a significant effect on the metabolism of amino acids in the body.
- Thiamine is a vitamin that affects the metabolism of carbohydrates and proteins. It affects the functioning of almost all organs.
- Vitamin B2 is a substance that helps accelerate metabolic processes. Promotes the binding of oxygen by cells.
- The remaining vitamins are presented in smaller quantities and are not able to influence the physiology of the body.
In addition to vitamins, the drink contains a sufficient amount of microelements. These are the following substances:
- calcium—affects connective tissue cells, ensuring their health and the normal course of physiological processes;
- magnesium - normalizes the functioning of cardiomycytes, restores heart rhythm. Also necessary in the formation of the bone skeleton and normalization of blood sugar levels;
- sodium - ensures the proper functioning of intercellular structures, normalizes the speed of impulses. This element has a beneficial effect on heart function and muscle contraction;
- Phosphorus is a necessary element that restores proper metabolism. Promotes regeneration of damage in the body;
- iron is an important trace element that is directly related to the amount of hemoglobin in the blood. It affects the normal course of all processes in the body.
Natural coffee, grown in environmentally friendly conditions, is not capable of having a harmful effect on the body. It does not contain harmful substances and oxidation products. But you must always adhere to the norm when drinking this drink.
Benefits of coffee for the body
Every year more and more beneficial properties are found in it. They manifest themselves to a greater extent with regular consumption of 3–4 cups of the drink.
Extends life
Numerous studies prove that regular moderate consumption of the drink improves the condition of the heart and blood vessels, reduces the risk of heart attacks and strokes . Scientists suggest that a temporary increase in blood pressure as a result of drinking coffee trains the circulatory system. Thus, the drink prolongs the life of men and women.
Invigorates and tones
Caffeine in the composition invigorates, relieves fatigue, improves memory.
Although, if you look at it, everything is a little more complicated than it seems. The mechanism of its action is due to the fact that it does not give a surge of strength, but simply prevents you from feeling tired . The brain has to use its untouchable energy reserves. When the effect of caffeine wears off, a person is immediately overcome by a wave of severe accumulated fatigue. Nevertheless, we can improve concentration and attention for some time with the help of a drink. You just need to give yourself the opportunity to rest after this.
Preventing diabetes
It has been proven to reduce tissue resistance to insulin, i.e., reduces the likelihood of diabetes mellitus . Decaffeinated coffee has the same effect. Flavonoids, polyphenols, lignans and other antioxidants are responsible for this.
Help the liver
Natural hepatoprotector. Extremely useful for the liver, especially if there are already any disorders (does not replace treatment). It has been proven to protect against the progression of cirrhosis to cancer .
Cancer Prevention
Scientists' assumptions that coffee can cause cancer have not been confirmed. Modern research, on the contrary, confirms that it reduces the risk of cancer of the prostate, endometrium of the uterus, liver and some other organs. Research in this direction continues.
Reduces the risk of Alzheimer's and Parkinson's diseases by 60%
Observations have shown that Parkinson's disease is much less common among coffee drinkers. In the case of Alzheimer's, it prevents the formation of pathological proteins that replace normal tissue in the brain. In the same way, the drink prevents the onset of senile dementia.
Other useful properties
- It has a mild diuretic effect, but with regular use the effect may be weakened.
- Stimulates intestinal function.
- Reduces uric acid levels, which is useful for gout.
- Slows down the formation of stones in cholelithiasis.
- Rich in antioxidants.
- Has anti-inflammatory effect.